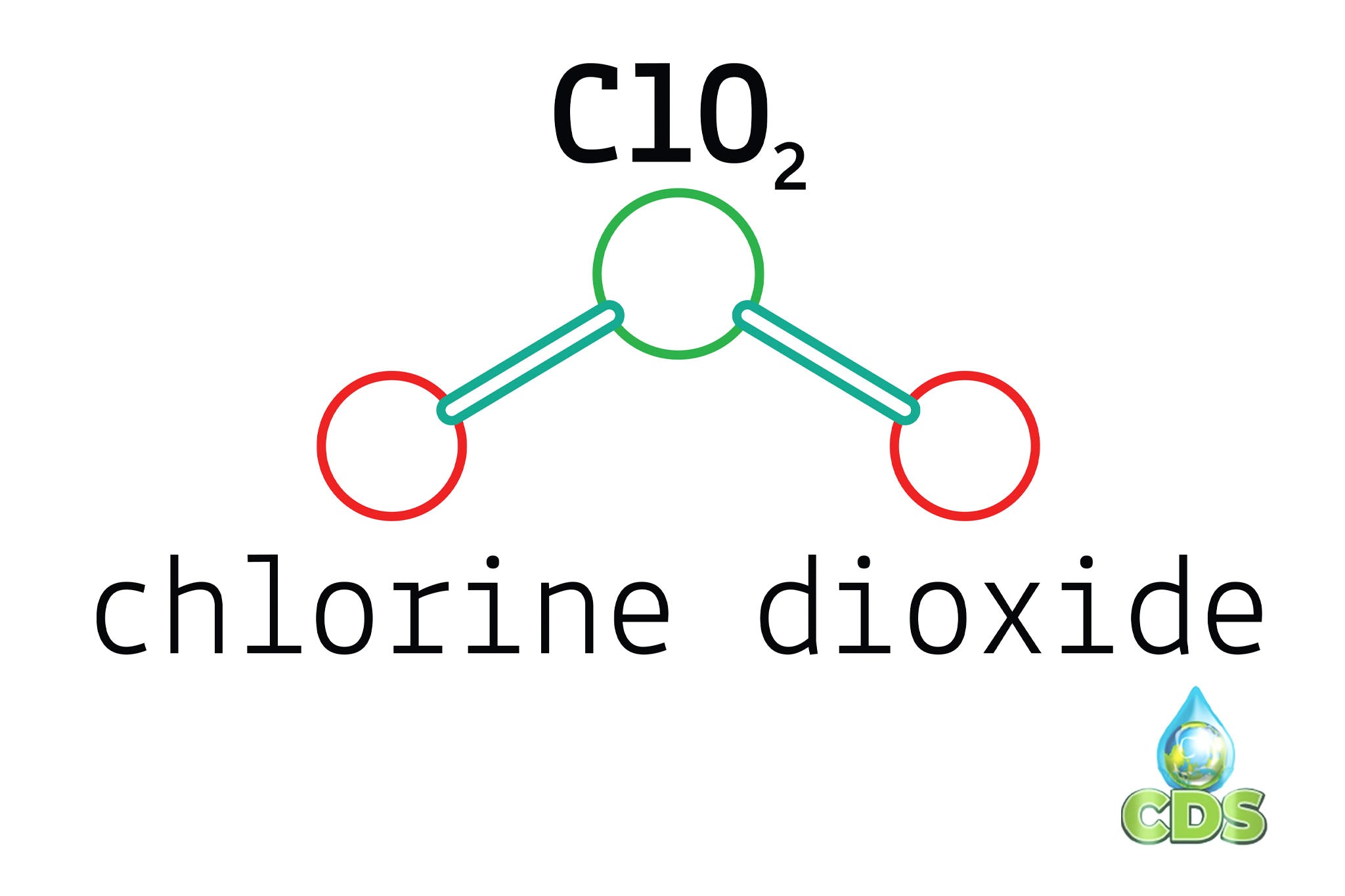
Chlorine Dioxide And Hydrogen Peroxide . The reaction of chlorine dioxide with hydrogen peroxide was studied in a well stirred batch reactor in a ph range of 3.60 to 5.07, which is. Chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. Greg simpson, phd, experimentally evaluates hydrogen peroxide vs chlorine dioxide to determine the dosage needed to oxidize sulfide. Chlorine dioxide (clo 2) and hydrogen peroxide (h 2 o 2) are two strong oxidizing agents with a broad antimicrobial activity offering a promising potential as contact surface sanitizers. One process for manufacturing chlorine dioxide involves reducing sodium chlorate with hydrogen peroxide. One process for manufacturing chlorine dioxide involves reducing sodium chlorate with hydrogen peroxide.
from chlorinedioxidesolution.ca
The reaction of chlorine dioxide with hydrogen peroxide was studied in a well stirred batch reactor in a ph range of 3.60 to 5.07, which is. One process for manufacturing chlorine dioxide involves reducing sodium chlorate with hydrogen peroxide. Greg simpson, phd, experimentally evaluates hydrogen peroxide vs chlorine dioxide to determine the dosage needed to oxidize sulfide. Chlorine dioxide (clo 2) and hydrogen peroxide (h 2 o 2) are two strong oxidizing agents with a broad antimicrobial activity offering a promising potential as contact surface sanitizers. One process for manufacturing chlorine dioxide involves reducing sodium chlorate with hydrogen peroxide. Chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media.
Chlorine Dioxide Formula chlorinedioxide
Chlorine Dioxide And Hydrogen Peroxide One process for manufacturing chlorine dioxide involves reducing sodium chlorate with hydrogen peroxide. The reaction of chlorine dioxide with hydrogen peroxide was studied in a well stirred batch reactor in a ph range of 3.60 to 5.07, which is. Chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. Greg simpson, phd, experimentally evaluates hydrogen peroxide vs chlorine dioxide to determine the dosage needed to oxidize sulfide. Chlorine dioxide (clo 2) and hydrogen peroxide (h 2 o 2) are two strong oxidizing agents with a broad antimicrobial activity offering a promising potential as contact surface sanitizers. One process for manufacturing chlorine dioxide involves reducing sodium chlorate with hydrogen peroxide. One process for manufacturing chlorine dioxide involves reducing sodium chlorate with hydrogen peroxide.
From pediaa.com
Difference Between Peroxide and Hydrogen Peroxide Definition Chlorine Dioxide And Hydrogen Peroxide One process for manufacturing chlorine dioxide involves reducing sodium chlorate with hydrogen peroxide. Chlorine dioxide (clo 2) and hydrogen peroxide (h 2 o 2) are two strong oxidizing agents with a broad antimicrobial activity offering a promising potential as contact surface sanitizers. Greg simpson, phd, experimentally evaluates hydrogen peroxide vs chlorine dioxide to determine the dosage needed to oxidize sulfide.. Chlorine Dioxide And Hydrogen Peroxide.
From www.slideserve.com
PPT Making Models of Atoms and Molecules PowerPoint Presentation Chlorine Dioxide And Hydrogen Peroxide The reaction of chlorine dioxide with hydrogen peroxide was studied in a well stirred batch reactor in a ph range of 3.60 to 5.07, which is. Greg simpson, phd, experimentally evaluates hydrogen peroxide vs chlorine dioxide to determine the dosage needed to oxidize sulfide. One process for manufacturing chlorine dioxide involves reducing sodium chlorate with hydrogen peroxide. Chlorine dioxide (clo. Chlorine Dioxide And Hydrogen Peroxide.
From cleanhospital.com
Chlorine Dioxide Horizontal Chlorine Dioxide And Hydrogen Peroxide Chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. Greg simpson, phd, experimentally evaluates hydrogen peroxide vs chlorine dioxide to determine the dosage needed to oxidize sulfide. One process for manufacturing chlorine dioxide involves reducing sodium chlorate with hydrogen peroxide. One process for manufacturing chlorine dioxide involves reducing sodium chlorate with hydrogen peroxide. The reaction. Chlorine Dioxide And Hydrogen Peroxide.
From pubs.acs.org
Electrochemical Production of Hydrogen Peroxide in Perchloric Acid Chlorine Dioxide And Hydrogen Peroxide Chlorine dioxide (clo 2) and hydrogen peroxide (h 2 o 2) are two strong oxidizing agents with a broad antimicrobial activity offering a promising potential as contact surface sanitizers. One process for manufacturing chlorine dioxide involves reducing sodium chlorate with hydrogen peroxide. Chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. One process for manufacturing. Chlorine Dioxide And Hydrogen Peroxide.
From www.pinterest.com
Oxidation Numbers Infographic Diagram Example Oxygen Stock Vector Chlorine Dioxide And Hydrogen Peroxide Chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. One process for manufacturing chlorine dioxide involves reducing sodium chlorate with hydrogen peroxide. The reaction of chlorine dioxide with hydrogen peroxide was studied in a well stirred batch reactor in a ph range of 3.60 to 5.07, which is. One process for manufacturing chlorine dioxide involves. Chlorine Dioxide And Hydrogen Peroxide.
From www.researchgate.net
(PDF) Virucidal Activity of Fogged Chlorine Dioxide and Hydrogen Chlorine Dioxide And Hydrogen Peroxide Greg simpson, phd, experimentally evaluates hydrogen peroxide vs chlorine dioxide to determine the dosage needed to oxidize sulfide. The reaction of chlorine dioxide with hydrogen peroxide was studied in a well stirred batch reactor in a ph range of 3.60 to 5.07, which is. One process for manufacturing chlorine dioxide involves reducing sodium chlorate with hydrogen peroxide. Chlorine dioxide can. Chlorine Dioxide And Hydrogen Peroxide.
From www.researchgate.net
(PDF) A Comparison of the Bleaching Effectiveness of Chlorine Dioxide Chlorine Dioxide And Hydrogen Peroxide Chlorine dioxide (clo 2) and hydrogen peroxide (h 2 o 2) are two strong oxidizing agents with a broad antimicrobial activity offering a promising potential as contact surface sanitizers. The reaction of chlorine dioxide with hydrogen peroxide was studied in a well stirred batch reactor in a ph range of 3.60 to 5.07, which is. One process for manufacturing chlorine. Chlorine Dioxide And Hydrogen Peroxide.
From chlorinedioxidesolution.ca
Chlorine Dioxide Formula chlorinedioxide Chlorine Dioxide And Hydrogen Peroxide The reaction of chlorine dioxide with hydrogen peroxide was studied in a well stirred batch reactor in a ph range of 3.60 to 5.07, which is. One process for manufacturing chlorine dioxide involves reducing sodium chlorate with hydrogen peroxide. Greg simpson, phd, experimentally evaluates hydrogen peroxide vs chlorine dioxide to determine the dosage needed to oxidize sulfide. Chlorine dioxide can. Chlorine Dioxide And Hydrogen Peroxide.
From www.simplymedsonline.co.uk
Buy Care Hydrogen Peroxide Solution 9 200ml Online Chlorine Dioxide And Hydrogen Peroxide Greg simpson, phd, experimentally evaluates hydrogen peroxide vs chlorine dioxide to determine the dosage needed to oxidize sulfide. One process for manufacturing chlorine dioxide involves reducing sodium chlorate with hydrogen peroxide. Chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. Chlorine dioxide (clo 2) and hydrogen peroxide (h 2 o 2) are two strong oxidizing. Chlorine Dioxide And Hydrogen Peroxide.
From onenessdrops.com
Understanding Chlorine Dioxide The Science Behind Water Purification Chlorine Dioxide And Hydrogen Peroxide Chlorine dioxide (clo 2) and hydrogen peroxide (h 2 o 2) are two strong oxidizing agents with a broad antimicrobial activity offering a promising potential as contact surface sanitizers. One process for manufacturing chlorine dioxide involves reducing sodium chlorate with hydrogen peroxide. Chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. The reaction of chlorine. Chlorine Dioxide And Hydrogen Peroxide.
From www.ingridscience.ca
Hydrogen peroxide chemistry ingridscience.ca Chlorine Dioxide And Hydrogen Peroxide One process for manufacturing chlorine dioxide involves reducing sodium chlorate with hydrogen peroxide. One process for manufacturing chlorine dioxide involves reducing sodium chlorate with hydrogen peroxide. Greg simpson, phd, experimentally evaluates hydrogen peroxide vs chlorine dioxide to determine the dosage needed to oxidize sulfide. Chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. Chlorine dioxide. Chlorine Dioxide And Hydrogen Peroxide.
From www.safesol.co.uk
Biofilm Chlorine Dioxide Versus HuwaSan (silver stabilised hydrogen Chlorine Dioxide And Hydrogen Peroxide One process for manufacturing chlorine dioxide involves reducing sodium chlorate with hydrogen peroxide. Chlorine dioxide (clo 2) and hydrogen peroxide (h 2 o 2) are two strong oxidizing agents with a broad antimicrobial activity offering a promising potential as contact surface sanitizers. Chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. Greg simpson, phd, experimentally. Chlorine Dioxide And Hydrogen Peroxide.
From www.indiamart.com
Hydrogen Peroxide at Rs 55/kg Gacl Hydrogen Peroxide in Gurgaon ID Chlorine Dioxide And Hydrogen Peroxide One process for manufacturing chlorine dioxide involves reducing sodium chlorate with hydrogen peroxide. Chlorine dioxide (clo 2) and hydrogen peroxide (h 2 o 2) are two strong oxidizing agents with a broad antimicrobial activity offering a promising potential as contact surface sanitizers. The reaction of chlorine dioxide with hydrogen peroxide was studied in a well stirred batch reactor in a. Chlorine Dioxide And Hydrogen Peroxide.
From www.mdpi.com
Catalysts Free FullText Production of Chlorine Dioxide Using Chlorine Dioxide And Hydrogen Peroxide Chlorine dioxide (clo 2) and hydrogen peroxide (h 2 o 2) are two strong oxidizing agents with a broad antimicrobial activity offering a promising potential as contact surface sanitizers. One process for manufacturing chlorine dioxide involves reducing sodium chlorate with hydrogen peroxide. Chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. Greg simpson, phd, experimentally. Chlorine Dioxide And Hydrogen Peroxide.
From plantcaretoday.com
How To Help You Plants With Hydrogen Peroxide Mixing Charts Chlorine Dioxide And Hydrogen Peroxide The reaction of chlorine dioxide with hydrogen peroxide was studied in a well stirred batch reactor in a ph range of 3.60 to 5.07, which is. Greg simpson, phd, experimentally evaluates hydrogen peroxide vs chlorine dioxide to determine the dosage needed to oxidize sulfide. One process for manufacturing chlorine dioxide involves reducing sodium chlorate with hydrogen peroxide. Chlorine dioxide can. Chlorine Dioxide And Hydrogen Peroxide.
From www.indiamart.com
Stabilized Chlorine Dioxide Solution at Rs 200/kg Liquid Chlorine Chlorine Dioxide And Hydrogen Peroxide One process for manufacturing chlorine dioxide involves reducing sodium chlorate with hydrogen peroxide. One process for manufacturing chlorine dioxide involves reducing sodium chlorate with hydrogen peroxide. The reaction of chlorine dioxide with hydrogen peroxide was studied in a well stirred batch reactor in a ph range of 3.60 to 5.07, which is. Chlorine dioxide (clo 2) and hydrogen peroxide (h. Chlorine Dioxide And Hydrogen Peroxide.
From www.researchgate.net
(PDF) Sterilization of hydrogen peroxide resistant bacterial spores Chlorine Dioxide And Hydrogen Peroxide One process for manufacturing chlorine dioxide involves reducing sodium chlorate with hydrogen peroxide. The reaction of chlorine dioxide with hydrogen peroxide was studied in a well stirred batch reactor in a ph range of 3.60 to 5.07, which is. One process for manufacturing chlorine dioxide involves reducing sodium chlorate with hydrogen peroxide. Greg simpson, phd, experimentally evaluates hydrogen peroxide vs. Chlorine Dioxide And Hydrogen Peroxide.
From segudanggambar875.blogspot.com
Hydrogen Peroxide And Manganese Dioxide Balanced Equation Hydrogen Chlorine Dioxide And Hydrogen Peroxide The reaction of chlorine dioxide with hydrogen peroxide was studied in a well stirred batch reactor in a ph range of 3.60 to 5.07, which is. One process for manufacturing chlorine dioxide involves reducing sodium chlorate with hydrogen peroxide. One process for manufacturing chlorine dioxide involves reducing sodium chlorate with hydrogen peroxide. Chlorine dioxide can be efficiently produced from hydrogen. Chlorine Dioxide And Hydrogen Peroxide.